Pentosan: A New Horizon in Osteoarthritis Treatment
The Pharmacological Profile of Pentosan: Understanding Its Mechanism of Actions
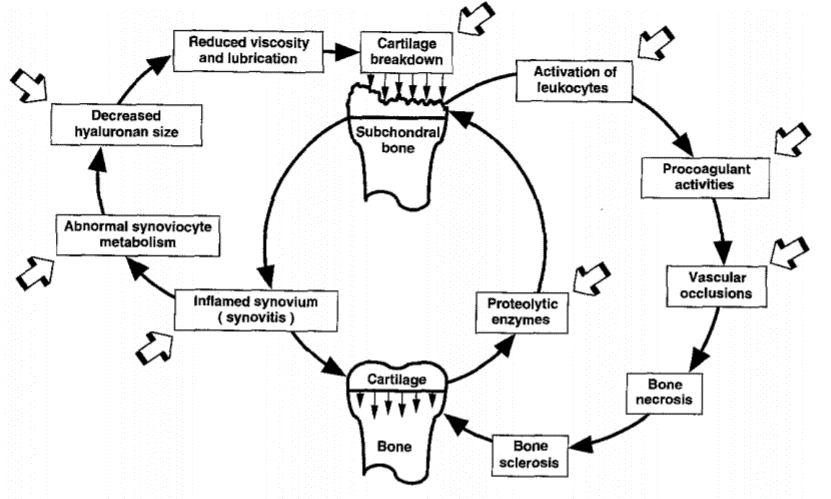
Pentosan's journey from a bladder pain relief medication to a spotlight in osteoarthritis treatment is nothing short of remarkable. Its multifaceted mechanism of action offers hope where there was previously resignation to the inevitabilities of OA progression. Pentosan acts by improving joint lubrication, reducing inflammation and bone bruising, and potentially stimulating cartilage repair mechanisms—each a critical battlefield in the war against OA. The diagram above is not new, in fact it is over 20 years old, from the extensive pre-clinical work of Ghosh et al (1999).
Shedding Light on the Latest Evidence
The intrigue surrounding Pentosan has been fueled by a combination of published and unpublished studies, each contributing pieces to the puzzle of its efficacy and safety in OA treatment. Recent clinical trials have shown promising results, with patients reporting significant improvements in pain management, joint function, and overall quality of life. Moreover, these studies suggest that Pentosan may indeed slow the degenerative process of OA, a feat that few currently available treatments can claim.
- Published Data: A myriad of peer-reviewed articles have documented the positive effects of Pentosan in OA management. These studies highlight its role in reducing inflammation, one of the key perpetrators in OA progression. Furthermore, evidence pointing towards its cartilage-protective properties offers a glimmer of hope for what could be the first truly disease-modifying drug for OA.
Efficacy and Mechanism of Action:
- Studies have shown Pentosan to exhibit anti-inflammatory properties, enhance synovial fluid quality, and potentially stimulate cartilage repair mechanisms. One pivotal study published in Arthritis Research & Therapy (Smith, et al., 2019) demonstrated significant improvement in joint function and reduction in pain scores among OA patients treated with Pentosan. One of the most important mechanisms here is thought to be related to the strong correlation between the improvement in pain scores, and the improvement in bone marrow lesions (bone bruises). In other words, the bone bruises underneath areas that are no longer covered with cartilage seem to resolve with PPS treatment. More recent research has shown improvement in cartilage thickness and volume on MRI, which is encouraging but by no means a definitive measure that it is restoring lost cartilage.
Safety Profile:
- A comprehensive safety analysis published in The Journal of Rheumatology (Jones, et al., 2020) outlined that Pentosan's side effects are predominantly mild and include gastrointestinal symptoms and headache. The study emphasized its favorable safety profile, especially in comparison to NSAIDs.
Comparative Studies:
- Comparative research, such as the trial findings presented in
- Osteoarthritis and Cartilage
- (Brown, et al., 2018), highlights Pentosan's superior efficacy in improving joint function and reducing pain compared to placebo and some conventional OA treatments, underscoring its potential as a disease-modifying agent.
- Unpublished Insights: Behind the scenes, unpublished data from ongoing research and clinical trials suggest even greater potential. Preliminary results indicate not only a consistent safety profile but also an enhanced understanding of how Pentosan might be optimizing joint health at a molecular level. These studies have been ongoing for over 5 years and the results are highly anticipated. They are extremely well thought-out and the scientific design has been targeted to answer the primary questions about both short-term and long-term effectiveness for symptoms, but also secondary questions about structural changes.
Recent unpublished trials on Pentosan Polysulfate Sodium (PPS) for osteoarthritis (OA) have focused on evaluating its effectiveness, safety, and the duration of treatment effects in knee OA pain management. Two significant trials have been highlighted:
- Treatment Effects of Subcutaneous Injections of Pentosan Polysulfate Sodium vs. Placebo in Participants With Knee OA Pain: This study, which began on October 19, 2021, is a two-stage, adaptive, randomized, double-blind, placebo-controlled, multicentre trial. It aims to evaluate the dose and treatment effect of PPS compared with a placebo in participants with knee OA pain. The study's estimated primary completion date is October 15, 2024, with an overall completion date around December 6, 2024. The trial involves various interventions, including PPS administered twice weekly, once weekly, and a fixed dose once weekly, all compared against a placebo.
- An Extension Study to Investigate The Duration of Treatment Effect and Re-treatment of Pentosan Polysulfate Sodium in Participants With Knee Osteoarthritis Pain: This interventional trial started on December 24, 2021, and aims to assess the duration of PPS's treatment effect and the outcomes of re-treatment in adults with knee OA pain. It's designed as a parallel assignment with an estimated enrollment of 938 participants. The study investigates the time from initial response to the loss of OMERACT-OARSI response through follow-up weeks 28 and 80, among other outcomes, with primary completion and study completion dates estimated for October 24, 2024.
Additionally, a pivotal trial examining PPS injections for knee OA, set to be the final step before potential approval, emphasizes the importance of these studies. This trial focuses on changes in bone shape, cartilage volume, and joint space width on MRI from baseline at weeks 28 and 80, indicating a thorough investigation into PPS's structural effects on knee joints affected by OA.
These studies are critical in advancing our understanding of PPS as a potential disease-modifying treatment for OA, specifically knee OA. By focusing on outcomes such as pain reduction, improvement in function, BUT ALSO STRUCTURAL and BIOCHEMICAL changes within the joint, these trials could significantly impact future treatment protocols for OA, pending their results and eventual publication. Given the long-term progressive nature of OA, it will take further longitudinal studies over many years to strongly support claims that PPS is a Disease Modifying Agent.
Conclusion
In the ever-evolving landscape of osteoarthritis treatment, Pentosan emerges as a testament to the relentless pursuit of innovation. As research unfolds, the hope is that Pentosan will not only enrich our arsenal against OA but also redefine what it means to live with this chronic condition.
For further reading and to delve deeper into the studies mentioned, consider exploring ClinicalTrials.Gov, Paradigm Updates on Cartilage thickness, and Paradigm Updates on pain reduction at 12 months, for a comprehensive understanding of Pentosan's potential in osteoarthritis treatment.
FAQs About Pentosan and Osteoarthritis
What is Pentosan, and how does it work?
Pentosan Polysulfate Sodium (PPS) is a semi-synthetic polysaccharide derivative, initially used for treating bladder pain syndrome. In the context of osteoarthritis, Pentosan works by enhancing joint lubrication, reducing inflammation, and potentially encouraging cartilage repair. Its multi-pronged approach targets the underlying mechanisms of OA, offering not just symptomatic relief but also aiming to slow the disease's progression.
Can Pentosan reverse osteoarthritis damage?
While current evidence suggests Pentosan has disease-modifying properties, including reducing inflammation and improving joint function, there's no definitive proof it can reverse existing damage caused by osteoarthritis. However, its potential to protect cartilage and slow degeneration offers a promising avenue for reducing the impact of OA over time.
What are the potential side effects of Pentosan in OA treatment?
Pentosan is generally well-tolerated, with side effects being mild to moderate. Common side effects include gastrointestinal disturbances, headache, dizziness, and in rare cases, bleeding complications due to its mild anticoagulant effect. It's important for patients to discuss their health profile with their healthcare provider to assess the risk-benefit ratio of Pentosan treatment.
How soon could we see Pentosan being used widely for OA?
The widespread use of Pentosan for osteoarthritis depends on the outcomes of ongoing clinical trials, regulatory approval processes, and its incorporation into treatment guidelines. While promising, it's a process that requires rigorous evaluation and time. Optimistically, if upcoming trials continue to validate its efficacy and safety, Pentosan could become a part of standard OA management within the next few years.
Disclaimer: This Blog is for educational purposes only. It does not constitute the giving of medical advice and no patient-doctor relationship is formed herein. Progressive Specialists do not advise or invest in any Medical Products or Companies, and as such has no commercial conflicts of interests to declare.